Monomers and polymers worksheet answer key – Welcome to our comprehensive guide to monomers and polymers, complete with a detailed worksheet answer key. This resource will delve into the fascinating world of these building blocks of matter, exploring their structures, properties, and applications in everyday life.
Throughout this guide, we’ll uncover the fundamental concepts of monomers and polymers, providing clear definitions and engaging examples to enhance your understanding. Our worksheet answer key will serve as a valuable tool, reinforcing your knowledge and ensuring a thorough grasp of the subject matter.
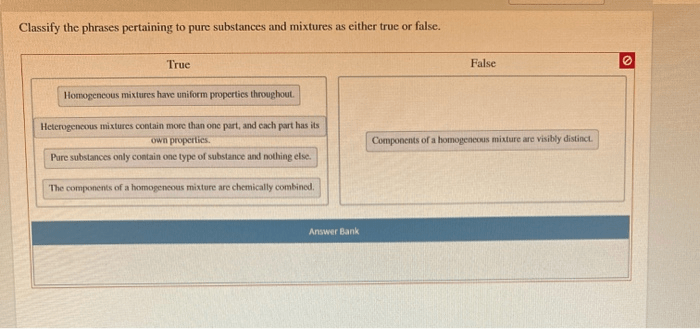
Monomers
Monomers are the basic building blocks of polymers. They are small, repeating units that can be linked together to form long chains. Monomers can be atoms, molecules, or ions. Some common examples of monomers include ethylene, propylene, and styrene. The structure of a monomer depends on the type of molecule it is.
For example, ethylene is a hydrocarbon with the formula C2H4. It has a double bond between the two carbon atoms. Propylene is also a hydrocarbon, but it has a different structure than ethylene. It has a single bond between the two carbon atoms and a methyl group attached to one of the carbon atoms.
Styrene is a hydrocarbon with the formula C8H8. It has a benzene ring with a vinyl group attached to it.
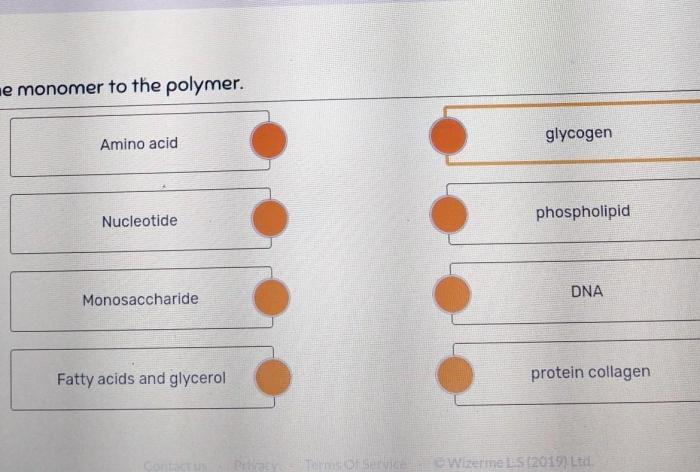
Polymers: Monomers And Polymers Worksheet Answer Key
Polymers are large molecules that are made up of many monomers. They can be natural or synthetic. Natural polymers include proteins, carbohydrates, and DNA. Synthetic polymers include plastics, rubber, and fibers. The structure of a polymer depends on the type of monomer it is made from and the way the monomers are linked together.
For example, polyethylene is a polymer made from ethylene monomers. The monomers are linked together in a long chain with a repeating
- CH2-CH2- structure. Polypropylene is a polymer made from propylene monomers. The monomers are linked together in a long chain with a repeating
- CH(CH3)-CH2- structure. Polystyrene is a polymer made from styrene monomers. The monomers are linked together in a long chain with a repeating
- CH(CH3)-CH2- structure.
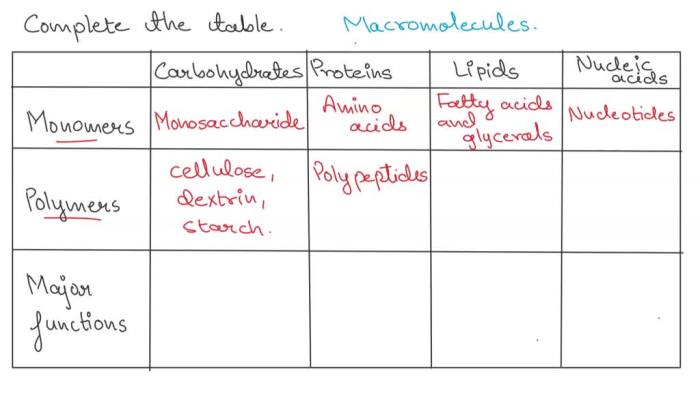
Polymerization
Polymerization is the process of linking monomers together to form polymers. There are two main types of polymerization: addition polymerization and condensation polymerization. Addition polymerization occurs when monomers are added to a growing polymer chain one at a time. Condensation polymerization occurs when monomers are linked together by the removal of a small molecule, such as water.
Monomers and Polymers in Everyday Life
Monomers and polymers are used in a wide variety of everyday products. Plastics are made from polymers, and they are used in everything from food packaging to car parts. Rubber is a polymer, and it is used in tires, hoses, and other products.
Fibers are polymers, and they are used in clothing, carpets, and other products.
Essential FAQs
What is a monomer?
A monomer is a single molecule that can combine with other identical molecules to form a polymer.
What is a polymer?
A polymer is a large molecule composed of repeating structural units called monomers.
What is the process of polymerization?
Polymerization is the chemical process by which monomers are joined together to form a polymer.
How are monomers and polymers used in everyday life?
Monomers and polymers are used in a wide range of products, including plastics, fabrics, and food additives.