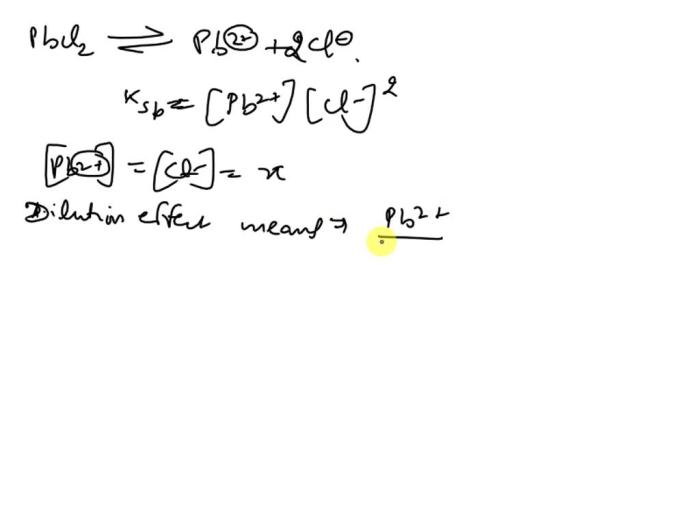Give the expression for the solubility product constant for pbcl2 – The solubility product constant (Ksp) of PbCl2 is a crucial concept in chemistry that governs the solubility of this ionic compound in aqueous solutions. Understanding the expression for Ksp is essential for predicting the behavior of PbCl2 in various chemical systems.
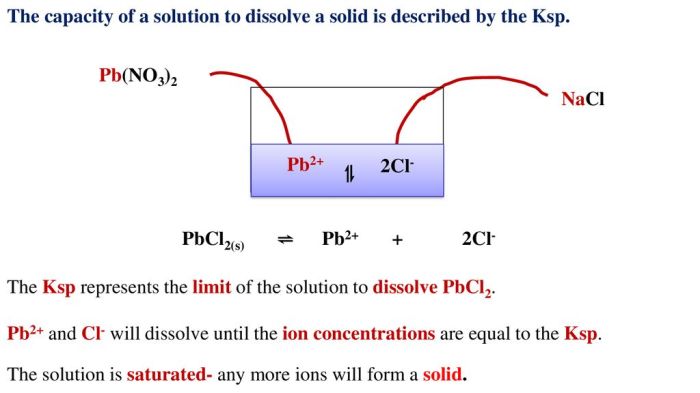
The chemical equation for the dissolution of PbCl2 is: PbCl2(s) <=> Pb2+(aq) + 2Cl-(aq). The equilibrium constant for this reaction is Ksp, which is expressed as Ksp = [Pb2+][Cl-]^2, where [Pb2+] and [Cl-] represent the molar concentrations of lead(II) and chloride ions in the solution, respectively.
Solubility Product Constant of PbCl2: Give The Expression For The Solubility Product Constant For Pbcl2

The solubility product constant (Ksp) is an equilibrium constant that describes the solubility of a solid ionic compound in a solvent. It is defined as the product of the molar concentrations of the ions of the compound in a saturated solution.
For example, the Ksp of PbCl2 is given by the expression:
Ksp = [Pb2+][Cl-]2
Factors Affecting Ksp of PbCl2
The value of Ksp for PbCl2 can be affected by several factors, including:
- Temperature:Ksp generally increases with increasing temperature, as higher temperatures favor the dissolution of the solid compound.
- Pressure:Ksp is not significantly affected by pressure for most ionic compounds, including PbCl2.
- Ionic strength:The presence of other ions in the solution can affect the Ksp of PbCl2. This is known as the common ion effect, which states that the solubility of a sparingly soluble salt is decreased by the addition of a common ion.
Applications of Ksp for PbCl2, Give the expression for the solubility product constant for pbcl2
The Ksp of PbCl2 has several applications in chemistry, including:
- Predicting solubility:The Ksp can be used to predict the solubility of PbCl2 in different solvents and at different temperatures.
- Determining the composition of saturated solutions:The Ksp can be used to determine the concentrations of Pb2+ and Cl- ions in a saturated solution of PbCl2.
- Designing precipitation reactions:The Ksp can be used to design precipitation reactions by controlling the concentrations of the ions involved.
Table of Ksp Values
The following table lists the Ksp values for PbCl2 and other common ionic compounds:
| Compound | Ksp (25 °C) | Units |
|---|---|---|
| PbCl2 | 1.2 x 10^-5 | M3 |
| CaCO3 | 8.7 x 10^-9 | M3 |
| AgCl | 1.8 x 10^-10 | M2 |
| BaSO4 | 1.1 x 10^-10 | M2 |
Q&A
What is the significance of Ksp for PbCl2?
Ksp for PbCl2 is important because it allows us to predict the solubility of PbCl2 in aqueous solutions and understand the factors that affect its dissolution.
How can Ksp be used to control the precipitation of PbCl2?
By manipulating Ksp, such as by adding common ions or changing the temperature, we can control the precipitation of PbCl2 and prevent its formation in unwanted situations.