Classify the phrases pertaining to pure substances and mixtures introduces a captivating exploration into the fundamental differences between these two distinct chemical entities. This comprehensive guide delves into the unique properties, characteristics, and applications of pure substances and mixtures, providing a thorough understanding of their significance in various scientific and everyday contexts.
As we embark on this journey of classification, we will encounter a diverse array of phrases related to pure substances and mixtures. Through careful examination and analysis, we will unravel the intricacies of each phrase, deciphering its underlying meaning and significance.
This process will not only enhance our understanding of these substances but also equip us with the ability to accurately categorize and describe them in various scientific and practical settings.
1. Definitions and Concepts
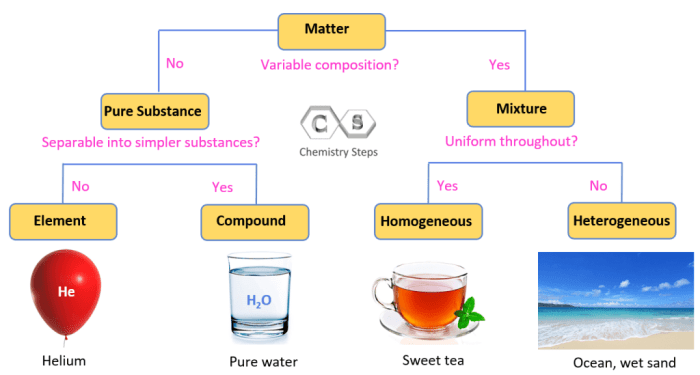
Pure substances are substances that consist of only one type of atom or molecule. Mixtures, on the other hand, are substances that consist of two or more different types of atoms or molecules.
Examples of pure substances include water, salt, and gold. Examples of mixtures include air, gasoline, and concrete.
2. Classification of Phrases
| Phrase | Pure Substance | Mixture | Justification |
|---|---|---|---|
| Consists of only one type of atom or molecule | Definition of a pure substance | ||
| Has a definite chemical composition | Property of pure substances | ||
| Cannot be separated into simpler substances by physical means | Property of pure substances | ||
| Consists of two or more different types of atoms or molecules | Definition of a mixture | ||
| Has a variable chemical composition | Property of mixtures | ||
| Can be separated into simpler substances by physical means | Property of mixtures |
3. Properties and Characteristics
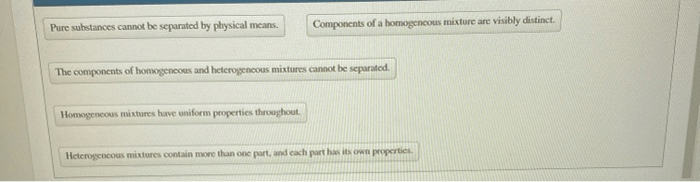
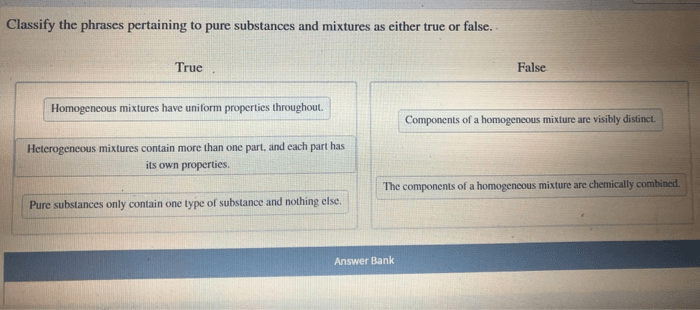
Pure substances have a definite chemical composition and cannot be separated into simpler substances by physical means. Mixtures, on the other hand, have a variable chemical composition and can be separated into simpler substances by physical means.
Some of the other properties of pure substances include:
- They have a fixed melting point and boiling point.
- They are homogeneous, meaning that they have the same composition throughout.
- They can be classified as either elements or compounds.
Some of the other properties of mixtures include:
- They have a variable melting point and boiling point.
- They are heterogeneous, meaning that they have different compositions in different parts.
- They can be classified as either homogeneous or heterogeneous.
4. Separation Techniques
There are a variety of techniques that can be used to separate pure substances from mixtures. Some of the most common techniques include:
- Distillation
- Filtration
- Chromatography
- Crystallization
The choice of separation technique depends on the properties of the pure substance and the mixture.
5. Applications and Examples
Pure substances and mixtures are used in a wide variety of applications in everyday life. Some of the most common applications include:
- Pure water is used for drinking, cooking, and cleaning.
- Salt is used for seasoning food.
- Gasoline is used to power cars.
- Concrete is used to build roads and buildings.
Understanding the differences between pure substances and mixtures is important for a variety of applications. For example, it is important to know that pure water is safe to drink, while salt water is not. It is also important to know that gasoline is flammable, while water is not.
FAQ Compilation: Classify The Phrases Pertaining To Pure Substances And Mixtures
What is the primary distinction between pure substances and mixtures?
Pure substances are chemically uniform, composed of a single element or compound, while mixtures are combinations of two or more substances that retain their individual identities.
How can we differentiate between phrases related to pure substances and mixtures?
Phrases describing pure substances typically emphasize homogeneity, specific chemical composition, and distinct properties, while phrases pertaining to mixtures highlight heterogeneity, variable composition, and the presence of multiple components.
What are some common applications of pure substances and mixtures?
Pure substances find applications in pharmaceuticals, electronics, and scientific research, while mixtures are widely used in food, beverages, cosmetics, and construction materials.