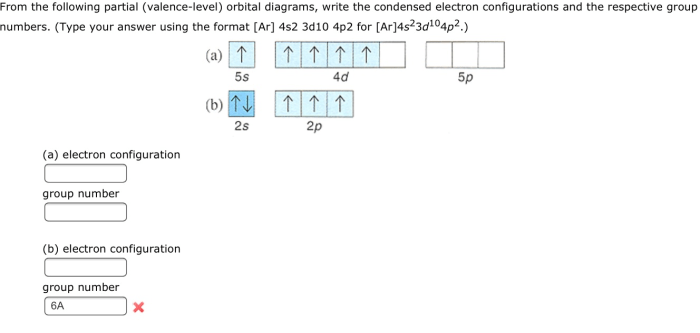
Partial valence level orbital diagram (PVLOD) unveils the intricate dance of electrons within molecules, providing a powerful tool for comprehending the fundamental principles of molecular bonding. This comprehensive exploration delves into the construction, interpretation, and applications of PVLOD, illuminating its significance in diverse scientific disciplines.
PVLOD empowers scientists to decipher the molecular blueprints of countless compounds, revealing insights into their geometry, reactivity, and properties. Its versatility extends from unraveling the complexities of chemical reactions to guiding the design of novel materials and pharmaceuticals.
Introduction to Partial Valence Level Orbital Diagram (PVLOD)
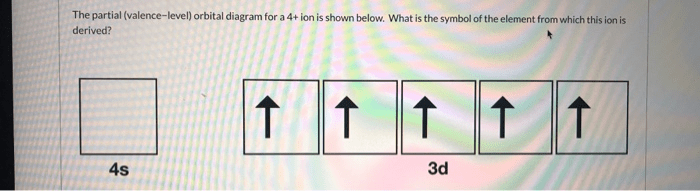
A partial valence level orbital diagram (PVLOD) is a graphical representation of the molecular orbitals of a molecule. It shows the energy levels of the orbitals, the number of electrons in each orbital, and the symmetry of the orbitals. PVLODs are useful for understanding molecular bonding because they provide a visual representation of the electronic structure of a molecule.
PVLODs can be used to analyze the bonding in a wide variety of molecules, including inorganic molecules, organic molecules, and organometallic compounds. They can be used to predict the properties of molecules, such as their bond lengths, bond angles, and reactivity.
Construction of a PVLOD
The first step in constructing a PVLOD is to identify the valence electrons of the molecule. The valence electrons are the electrons in the outermost shell of the atoms in the molecule. Once the valence electrons have been identified, the next step is to determine the molecular orbitals of the molecule.
Molecular orbitals are formed by the combination of atomic orbitals. The atomic orbitals of the atoms in a molecule interact with each other to form molecular orbitals. The molecular orbitals have different energies and symmetries. The energy of a molecular orbital is determined by the energy of the atomic orbitals that interact to form it.
The symmetry of a molecular orbital is determined by the symmetry of the atomic orbitals that interact to form it.
Once the molecular orbitals have been determined, the next step is to fill the molecular orbitals with electrons. The electrons are filled into the molecular orbitals in order of increasing energy. The number of electrons in each molecular orbital is determined by the number of valence electrons in the molecule.
Interpretation of a PVLOD
A PVLOD can be used to interpret the bonding in a molecule. The bond order of a bond is determined by the number of electrons in the bonding molecular orbital. The molecular geometry of a molecule is determined by the symmetry of the molecular orbitals.
The electron distribution in a molecule is determined by the number of electrons in each molecular orbital.
PVLODs can be used to predict the properties of molecules. For example, the bond order of a bond can be used to predict the strength of the bond. The molecular geometry of a molecule can be used to predict the shape of the molecule.
The electron distribution in a molecule can be used to predict the reactivity of the molecule.
Applications of PVLOD
PVLODs are used in a wide variety of fields, including chemistry, materials science, and biochemistry. In chemistry, PVLODs are used to understand the bonding in molecules. In materials science, PVLODs are used to design new materials with specific properties. In biochemistry, PVLODs are used to understand the structure and function of biological molecules.
PVLODs are a powerful tool for understanding the electronic structure of molecules. They can be used to predict the properties of molecules and to design new materials with specific properties.
Limitations of PVLOD, Partial valence level orbital diagram
PVLODs are a useful tool for understanding the electronic structure of molecules, but they have some limitations. PVLODs are only applicable to molecules that have a closed-shell electronic configuration. PVLODs cannot be used to analyze the bonding in molecules that have unpaired electrons.
Another limitation of PVLODs is that they do not take into account the effects of electron correlation. Electron correlation is the interaction between electrons. Electron correlation can affect the energy and symmetry of the molecular orbitals. In some cases, electron correlation can lead to significant changes in the properties of a molecule.
Despite their limitations, PVLODs are a valuable tool for understanding the electronic structure of molecules. They can be used to predict the properties of molecules and to design new materials with specific properties.
Query Resolution
What is the significance of PVLOD in understanding molecular bonding?
PVLOD provides a visual representation of the molecular orbitals, which are key to understanding the bonding patterns and properties of molecules.
How is a PVLOD constructed?
PVLOD construction involves identifying valence electrons, determining molecular orbitals, and considering symmetry and energy levels.
What information can be obtained from a PVLOD?
PVLODs provide insights into bond order, molecular geometry, electron distribution, and other properties of molecules.
What are the limitations of PVLOD?
PVLODs may not be applicable to all types of molecules and have certain assumptions, necessitating alternative methods for complex systems.