Embark on a comprehensive learning journey with our meticulously crafted Unit 3 AP Chemistry Practice Test. This test is designed to provide you with an in-depth understanding of the fundamental concepts covered in Unit 3, equipping you with the knowledge and skills necessary to excel in your AP Chemistry exam.
Our practice test delves into the intricacies of thermodynamics, equilibrium, kinetics, electrochemistry, and nuclear chemistry. Through a series of challenging questions, you will explore the concepts of entropy, spontaneity, equilibrium constants, reaction rates, oxidation-reduction reactions, and nuclear decay.
Thermodynamics
Thermodynamics is the study of energy and its transformations. It provides a framework for understanding the behavior of matter and energy in chemical reactions and other processes.
Entropy
Entropy is a measure of disorder or randomness in a system. In chemical reactions, entropy increases as the products become more disordered than the reactants.
Exothermic and Endothermic Reactions
Exothermic reactions release heat, while endothermic reactions absorb heat. The change in enthalpy (ΔH) is the amount of heat released or absorbed in a reaction.
Enthalpy and Free Energy
Enthalpy is a measure of the total energy of a system. Free energy is a measure of the energy available to do work. The spontaneity of a reaction can be predicted based on the change in free energy (ΔG).
Equilibrium
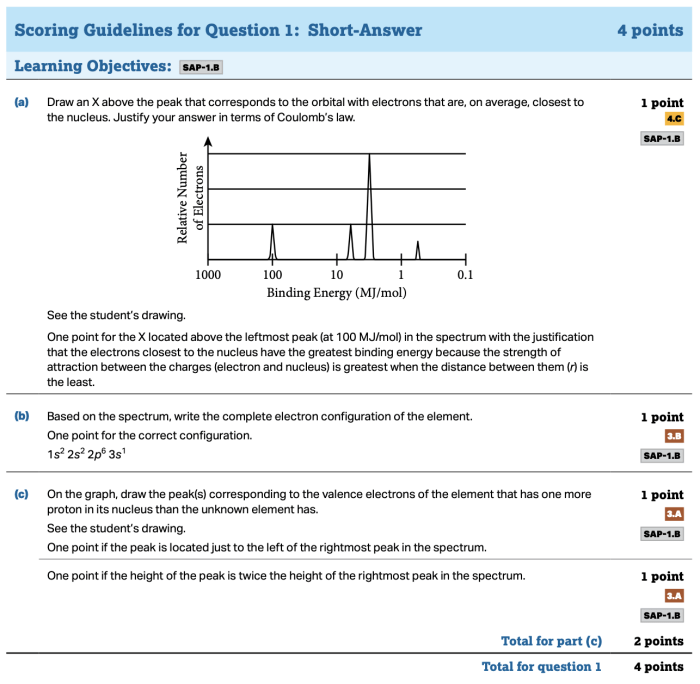
Chemical equilibrium is a state in which the forward and reverse reactions occur at equal rates, resulting in no net change in the concentrations of the reactants and products.
Factors Affecting Equilibrium
- Concentration of reactants and products
- Temperature
- Pressure (for gas reactions)
Equilibrium Constants
- Kp: Equilibrium constant expressed in terms of partial pressures
- Kc: Equilibrium constant expressed in terms of molar concentrations
- Kw: Ion product constant for water
Shifting Equilibrium
Equilibrium can be shifted by changing the concentration, temperature, or pressure of the system.
Kinetics: Unit 3 Ap Chemistry Practice Test
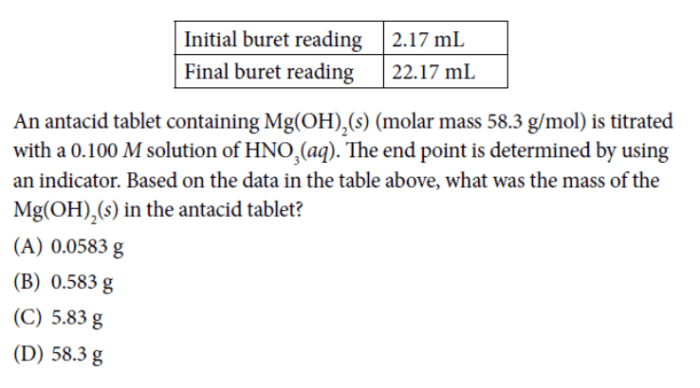
Kinetics is the study of reaction rates and the factors that affect them.
Reaction Rate
Reaction rate is the change in concentration of reactants or products per unit time.
Factors Affecting Reaction Rate
- Concentration of reactants
- Temperature
- Surface area of reactants
- Presence of a catalyst
Rate Laws
Rate laws express the relationship between the reaction rate and the concentrations of the reactants.
Rate-Determining Step
The rate-determining step is the slowest step in a reaction mechanism and determines the overall reaction rate.
Electrochemistry
Electrochemistry is the study of chemical reactions that involve the transfer of electrons.
Oxidation-Reduction Reactions
Oxidation-reduction reactions involve the transfer of electrons between atoms or ions.
Electrochemical Cells
- Voltaic cells: Convert chemical energy into electrical energy
- Electrolytic cells: Convert electrical energy into chemical energy
Electrochemical Applications
- Batteries
- Fuel cells
Nuclear Chemistry
Nuclear chemistry is the study of the structure and reactions of atomic nuclei.
Structure of the Atom
Atoms consist of a nucleus (protons and neutrons) and electrons orbiting the nucleus.
Nuclear Reactions, Unit 3 ap chemistry practice test
- Nuclear fission: Splitting of a heavy nucleus into smaller nuclei
- Nuclear fusion: Combining of light nuclei into a heavier nucleus
Radioactive Decay
Radioactive decay is the spontaneous emission of particles or energy from an unstable nucleus.
Half-Life
Half-life is the time it takes for half of a radioactive sample to decay.
Nuclear Applications
- Medicine (e.g., cancer treatment, medical imaging)
- Energy production (e.g., nuclear power plants)
Expert Answers
What topics are covered in Unit 3 AP Chemistry?
Unit 3 AP Chemistry covers thermodynamics, equilibrium, kinetics, electrochemistry, and nuclear chemistry.
How many questions are on the Unit 3 AP Chemistry Practice Test?
The number of questions on the practice test will vary depending on the specific test you are taking.
Is the Unit 3 AP Chemistry Practice Test timed?
Some practice tests are timed, while others are not. Be sure to check the instructions for the specific test you are taking.